FAQ
Ordering from foreign users and Oversea Shipment
How can we obtain a JP Reference Standard?
General tests, Processes and apparatus, 9.01 Reference standards (1)
The JP Reference Standards listed in JP, General tests, Processes and apparatus, 9.01 Reference standards (1), are produced and distributed by PMRJ. For ordering them, please visit the PMRJ Reference Standards Online Store.
For more details, please see “How to Order”.
General tests, Processes and apparatus, 9.01 Reference standards (2)
The JP Reference Standards, listed in JP, General tests, Processes and apparatus, 9.01 Reference standards (2), are produced and distributed by the National Institute of Infectious Diseases (NIID).
For ordering them, please contact the Murayama Branch of NIID
Tel : +81-425-61-0771
Fax : +81-425-65-3315
Please advise me how to place an order.
The steps for ordering PMRJ reference standards are as below.
1. Visit PMRJ Reference Standards Online Store.
2. Please fill in the required items of the quotation form.
3. Upon receipt of your inquiry, we will send you our proforma invoice.
4. Please send us your purchase order subject to your acceptance of our Terms and Conditions.
5. Upon receipt of your purchase order, we will send you our order confirmation and invoice necessary for your remittance.
6. Please remit us the full invoice amount as soon as you receive our order confirmation and Invoice.
7. After our receipt of the full invoice amount, we will ship out your order subject to our securing the necessary cargo space.
How much is the shipping charge?
The shipping charges vary depending on the destination country, temperature zone,
and whether the item has a UN number.
Basically, we ship by CPT (Incoterm 2020).
Please confirm the actual shipping costs by placing an order through our online store.
How can overseas users know the availability for overseas shipping?
Some of the reference standards which are legally restricted cannot be shipped overseas. You can check the availability of the reference standards on our website or current catalog.
Can overseas users designate its own carrier?
PMRJ can accept user’s designated carrier in case of Ex-works (Incoterms 2020).
Questions and Answers on Storage of JP and Other Compendial Reference Standards
How should I store JP and other compendial reference standards?
After receipt of a reference standard, immediately store it at the specified temperature and use it as soon as possible. The storage temperature of each reference standard is noted in the Storage Conditions section of its leaflet and on the exterior label.
| Room Temperature | 1 – 30°C |
|---|---|
| ≤25°C | 1 – 25°C |
| Refrigerate (≤8°C) | 1 – 8°C |
| Freeze (≤−20°C) | ≤−20°C |
| Freeze (−20 to −30°C) | ≤−20°C. Avoid storage below −30°C because the container may not withstand such low temperatures. |
| Freeze (−80°C) | −80 ± 10°C |
Questions and Answers on Use of JP and Other Compendial Reference Standards
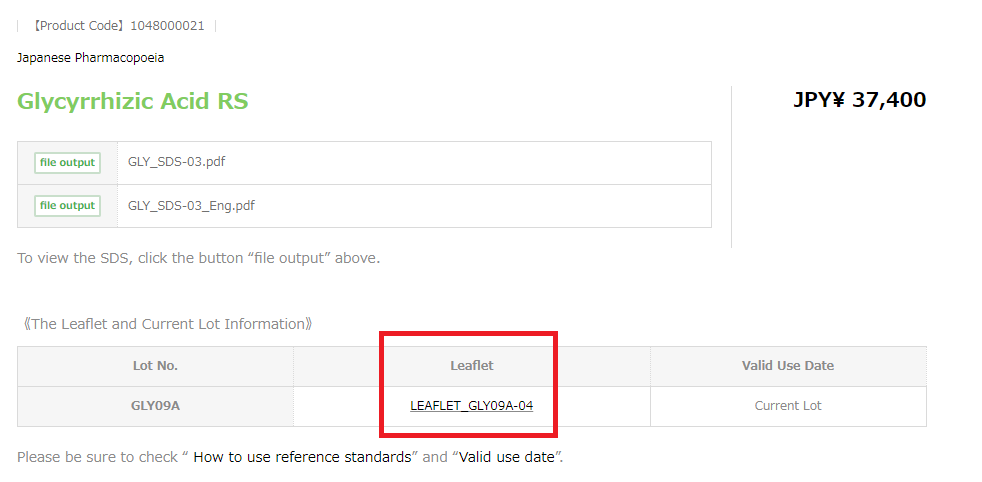
How can I obtain the leaflet for a reference standard?
The leaflet for each reference standard is available on the webpage for the reference standard of the PMRJ website .
Example: Glycyrrhizic Acid RS
Are the leaflets for previously purchased reference standards available on the website?
The leaflets for the current lots of reference standards are available on the website. When a lot is replaced after April 1, 2024, the leaflet for the previous lot is also available on the website. However, leaflets for previous lots depleted by the end of March, 2024 are not available on the website. If the leaflet for a reference standard that was purchased in the past is not available on the website, please contact us by using the “Questions and Comments Form.”
What is the control number shown on the label?
The control number is a product control number used by PMRJ. The control number is different from the lot number.
Can the certificate of analysis for a JP or other compendial reference standard be provided with the reference standard?
The certificate of analysis is not provided and also the test results are not disclosed.
Can I obtain the quality specifications for a JP or other compendial reference standard?
The quality specifications are not disclosed.
For some JP and other compendial reference standards, the user is directed to dry the reference standard before use in tests requiring that reference standard. Have such reference standards already been dried by PMRJ before shipment to the user?
Such reference standards have not been dried before shipment. The user should dry, as directed, the proper quantity of reference standard before use.
Can you tell me about the loss on drying or water content value of JP and other compendial reference standards?
When “amount (mg) of ABC reference standard taken, calculated on the dried basis” or “amount (mg) of XYZ reference standard taken, calculated on the anhydrous basis” is prescribed in a calculation formula to determine an assay value in a test method specified in an official compendium such as JP, the analyst should determine the loss on drying or water content of the reference standard, and calculate the amount of reference standard taken on the dried or anhydrous basis.
However, if the Correction Information section of the reference standard leaflet contains a loss on drying or water content value, the amount taken may be converted to the amount calculated on the dried or anhydrous basis by using the value given in the leaflet.
Can you tell me about the assigned value (e.g., purity and potency) of the reference standards?
The content / potency of the reference standards for quantitative tests is assigned in compliance with intended uses specified in JP and other compendial monographs. In general, the purity (%) based on the mass balance method for chemical reference standards, the potency (μg/mg) of activity for antibiotics reference standards, and the potency (units of biological activity) or the protein content for biological reference standards are assigned. In principle, the collaborative studies for determining the assigned values are conducted by more than three laboratories including PMRJ and external testing laboratories.
How is the purity of reference standard (RS) calculated by the mass balance method?
The purity of the RS is calculated by the mass balance method. Generally, mass balance purity is calculated by deducting the measured levels of impurities (including residue on ignition, residual solvents, water content, and related substances) from 100.0%.
When the related substances % has been determined by chromatography rather than being based on mass fraction, purity is calculated using Formula A.
Formula A: Purity (as is)
= {100% − (residue on ignition % + residual solvents % + water content %)} × (100% − related substances %) / 100
However, for JPRSs, when JP Monograph quantitative tests that use the JPRS contain directions to dry the RS or to convert the RS value by calculating on the dried basis, calculating on the anhydrous basis, or calculating on the anhydrous and residual solvent–free basis, purity is determined by subtracting the measured impurities from 100.0% after drying the RS or performing the specified conversion. For example, after calculating the RS value on the anhydrous basis, purity is calculated using Formula B.
Formula B: Purity (anhydrous substance)
= {100% − (residue on ignition % + residual solvents %)} × (100% − related substances %) / 100
The impurities that are subtracted differ depending upon the specificity of JP Official Monograph quantitative tests that use the RS. For example, regarding RSs used in assays performed by ultraviolet-visible spectrophotometry, substances having the same absorption as the main component at the specified wavelength are not regarded as impurities. Likewise, regarding RSs used in assays performed under conditions that cannot separate optical isomers, optical isomers are not regarded as impurities.
What are the correction factors in the leaflets for some reference standards?
When some reference standards are to be used in quantitative tests specified in official compendia, their purity is calculated by the mass balance method, etc., and shown as a correction factor. In determining whether to provide such correction factors, the test methods in which the reference standards are used are taken into consideration.
Example of correction factors:
Purity 99.99% → correction factor: 1.000 (Assay (LC), Uniformity of dosage units (LC))
Purity 99.75% → correction factor: 0.998 (Assay (LC), on the anhydrous basis)
As above, when a correction factor is provided in the Correction Information section of a leaflet, the quantitative tests in which the correction factor is to be used and the way of the specified conversion (e.g., on the anhydrous basis) are indicated following the correction factor, that is in parentheses. When the reference standard is used in any of those quantitative tests, the weighed amount of reference standard must be corrected by the correction factor. Different lots of the same reference standard can have different correction factors, so please be sure to check the leaflet for the reference standard lot that will be used.
How should I use correction factors?
If a correction factor is provided in the Correction Information section of the leaflet for a reference standard, correct the weighed amount of reference standard by multiplying it by the correction factor when the reference standard is used in the quantitative tests specified following the correction factor. If a correction factor is not provided, the reference standard is regarded as being 100.0%, and there is no need to correct the weighed amount.
Example: Excerpt from Japanese Pharmacopoeia Epitiostanol Reference Standard leaflet
• JP Mepitiostane: Purity (LC) and Assay (LC)
《Correction Information》
Water content: 1.5%
Correction factor: 0.990 (Assay (LC), on the anhydrous basis)
Mepitiostane Assay instructions in JP (excerpted)
= MS × (QT /QS) × 5 × 1.320
MS: Amount (mg) of Epitiostanol RS taken, calculated on the anhydrous basis
Correction method for using in Assay (LC) specified in JP Mepitiostane: The correction factor, 0.990, indicates the purity of anhydrous Epitiostanol Reference Standard. Therefore, first calculate the amount (mg) of Epitiostanol Reference Standard on the anhydrous basis by using the water content value of 1.5% given in the Correction Information section of the leaflet. Then, multiply that amount by the correction factor 0.990, and use the result as the MS value in the calculation formula. Inclusion of the above-mentioned steps in the JP calculation formula results in the following formula:
Amount (mg) of mepitiostane (C25H40O2S)
= Amount of reference standard actually weighed (mg) × (100 – 1.5)/ 100 × 0.990 × (QT / QS) × 5 × 1.320
Under what circumstances are correction factors provided?
Basically, when the purity of reference standard is ≥99.5%, its purity had been regarded as 100.0% according to the policy of JP. It is why not many chemical reference standards used in quantitative tests had been provided correction factors in the past. However, by following this policy, reference standards used in quantitative tests cause the error up to 0.5%. Therefore, it was decided that the policy changed to the following; Basically, the chemical reference standards used in quantitative tests are provided correction factors even if their purities are ≥99.5%, and the weighed amount of the reference standards must be corrected by the correction factor when the reference standards are used in any of those quantitative tests. The reference standards which have been newly established since the JP17th Edition Supplement I and the new lots of reference standards which have been released since 2019 have been provided their purity calculated by the mass balance method as the correction factors.
The leaflet specifies the quantitative tests in which the correction factor is to be used. Can the correction factor be used in quantitative tests that are not specified in the leaflet?
As explained in A10, correction factors are established based on purity, considering the test methods in which the reference standards are specified to be used. For this reason, correction factors must not be used in tests other than those specified in the leaflet. For example, a correction factor that is used in an assay by liquid chromatography cannot be used in an assay by ultraviolet-visible spectrophotometry.
Questions and Answers on Quality Assurance for JP and Other Compendial Reference Standards
To what extent is the quality of the reference standards assured?
The reference standards distributed by PMRJ are assured to be suitable for the tests in the official compendia in which their use is specified. The specified intended uses of each reference standard are given in the Intended Uses section of its leaflet. The quality of the reference standard cannot be assured if it is used in tests other than those specified in the leaflet.
Do the JP and other compendial reference standards have expiration dates?
The JP and other compendial reference standards do not have expiration dates like those assigned based on shelf-life for pharmaceuticals. Please refer “Reference Standards and Reference Materials Specified in the Japanese Pharmacopoeia〈G8-1-170〉” in the “General Information” section of JP.
On the other hand, valid use dates are assigned for the reference standards distributed by PMRJ. The valid use date assigned for the reference standards distributed by PMRJ is one year from the end of distributing a lot (excluding items specified separately). PMRJ regularly carries out verification tests in the stability monitoring programs on the reference standards to check the quality of each lot from the start of distribution until one year after the end of distribution. The valid use dates are guaranteed, only if the product is unopened and properly stored under the specified storage conditions. The current lot number being distributed can be found on the webpage for each reference standard in the PMRJ online store. Once the distribution of a lot ends, the valid use date for the lot can be found on the webpage in the PMRJ online store. For items specified separately, the details can be found on the webpage for each reference standard. Please be aware that the quality of a reference standard cannot be guaranteed if it has been stored after opening.
Please order only the required amount of the reference standards that can be used immediately and store the reference standards under the specified conditions after their receipt. Please use the reference standards as soon as possible even if it is still within the valid use date.
When performing tests specified in JP, can I use reference standards other than JP reference standards, e.g., USP or EP reference standards?
When use of a JP reference standard is directed in JP, the JP reference standard must be used. A reference standard other than the JP reference standard may not be used.
When a bioassay test method based on a JP reference standard is specified in an official monograph in JP, can that reference standard also be used as a reference standard in other test methods not present in the official monographs in JP, e.g., assay by HPLC?
JP reference standards have been established for use in tests specified in JP; their quality is assured only for those intended uses. The intended uses specified in JP are given in the leaflet for each reference standard. Please be aware that the quality of reference standards distributed by PMRJ cannot be assured if they are used in applications not specified in their leaflets.
What are the basic policies for establishing JP reference standards, and how is the quality of JP reference standards evaluated?
The basic policies for establishing JP reference standards and the parameters for evaluating their quality are given in “Reference Standards and Reference Materials Specified in the Japanese Pharmacopoeia〈G8-1-170〉” in the “General Information” section of JP. Please refer to that.
Others
–
–